Magstim获FDA批准:适用于美国青少年抑郁症患者
发布时间:2025-05-21 09:23:07 来源:本站 点击:276次
里程碑式进展
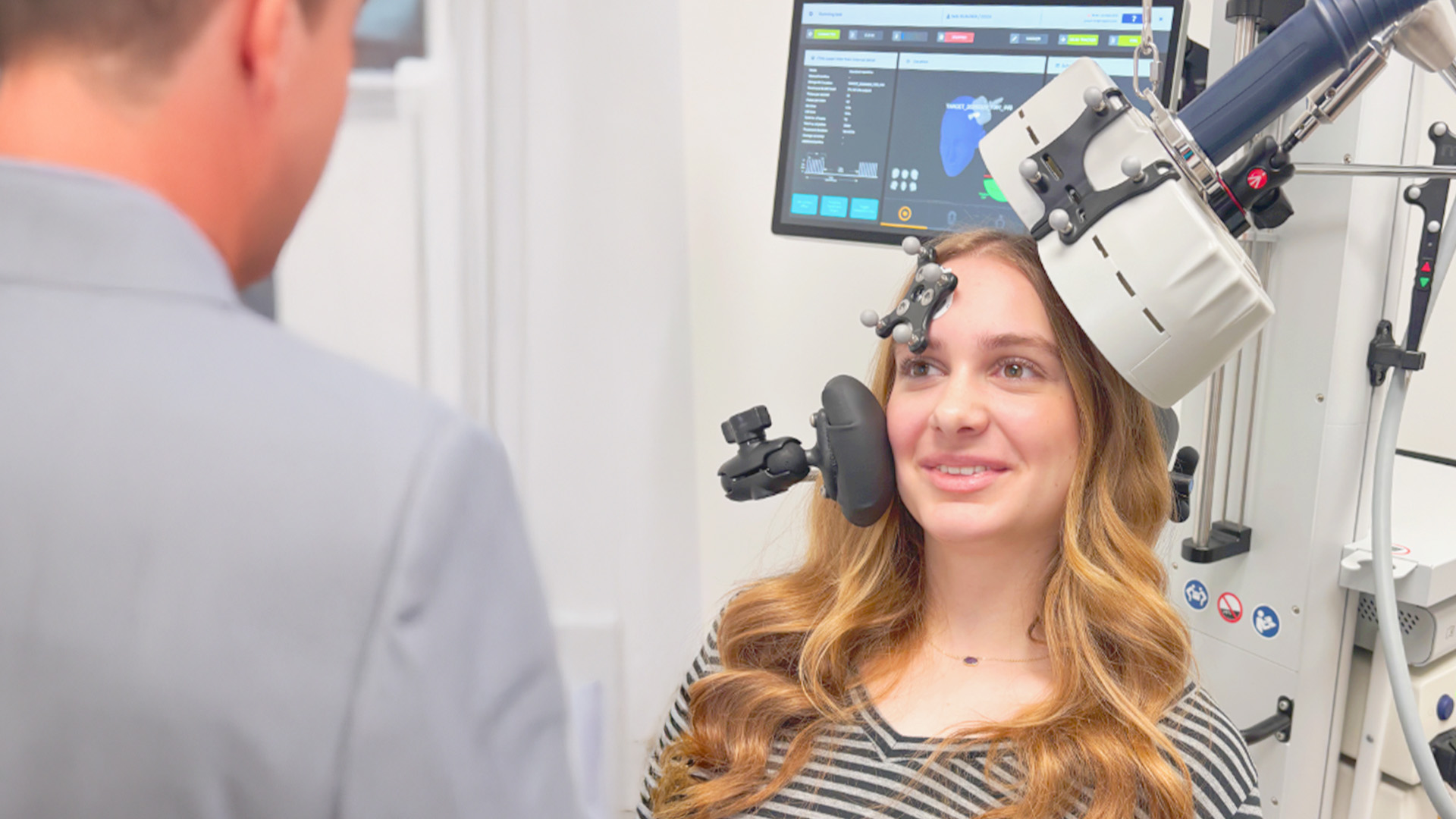
Magstim Horizon Inspire及3.0经颅磁刺激系统正式获得美国FDA批准,用于15-21岁青少年重性抑郁症(MDD)。这一突破性进展为青少年抑郁症患者提供了全新的非侵入性方案,标志着精神健康进入精准化时代。
青少年新选择
此次获批的TMS系统采用领先的神经调控技术,具有以下临床优势:
✓ 精准安全:非侵入式靶向刺激,无全身性副作用
✓ 疗效显著:针对传统疗法反应不佳的青少年患者
✓ 个性定制:可根据发育阶段调整参数
"这填补了青少年精神健康关键成长期的空白,"Magstim首席医疗官表示,"TMS为青少年提供了区别于传统药物的创新选择。"
开启精神健康新纪元
随着Horizon系列TMS系统获批,我们正推动青少年精神健康的范式变革:
• 为神经发育关键期提供安全干预方案
• 建立循证医学指导下的精准标准
• 完善从青少年到成人的全周期体系
免费线上演示预约
欢迎医疗专业人士点击此处预约系统演示,了解TMS如何改变青少年抑郁症应用与研究的格局。
适应症说明
▌成人患者(FDA批准)
• 难治性抑郁症(当前发作期对抗抑郁药反应不足)
• 强迫症(OCD)
▌青少年患者(15-21岁)
• 重性抑郁症(MDD)
Exciting News in Mental Health Treatment
Magstim Horizon Inspire and 3.0 Transcranial Magnetic Stimulation Systems have officially received FDA clearance for the treatment of Major Depressive Disorder (MDD) in adolescent patients, ages 15-21. This marks a significant milestone in mental health care, opening new doors for more effective, non-invasive treatment options tailored specifically for younger patients battling depression.
A Groundbreaking Advancement in Adolescent Care
The FDA clearance of the Horizon Inspire and 3.0 Transcranial Magnetic Stimulation systems represents a powerful step forward in offering hope and better outcomes for adolescents struggling with MDD. These advanced systems leverage leading Transcranial Magnetic Stimulation technology to deliver precise and effective treatment in a safe, non-invasive manner.
By providing a solution for adolescent patients, Magstim is helping to address the critical need for innovative mental health treatments during one of the most formative periods of life. Transcranial Magnetic Stimulation offers a unique alternative to traditional therapies, providing a personalized approach that can significantly improve the lives of young individuals experiencing depression.
Transforming the Future of Mental Health Treatment
With the clearance of these FDA-approved Horizon Transcranial Magnetic Stimulation systems, we are excited to be part of the movement to transform the landscape of mental health treatment for younger generations. This advancement is a crucial step in ensuring that adolescents have access to the best possible care as they navigate mental health challenges.
If you’re interested in learning more about how the Magstim Horizon Transcranial Magnetic Stimulation Systems can support adolescent MDD treatment, we invite you to book a free virtual demonstration today!
Click here to schedule your virtual demo: https://www.magstim.com/contact-us/
Note:
For Adults: Horizon 3.0 Transcranial Magnetic Stimulation Therapy Systems are indicated for the treatment of Major Depressive Disorder (MDD) in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode, as well as an adjunct for the treatment of adult patients suffering from Obsessive-Compulsive Disorder (OCD). For Adolescents: Horizon 3.0 Transcranial Magnetic Stimulation Therapy Systems are indicated as an adjunct for the treatment of MDD in adolescent patients (age 15-21).
北京华泰长润科技发展有限公司相关业务:
进口磁刺激仪, 进口经颅磁, 经颅磁刺激 科研, 导航经颅磁, 外国经颅磁刺激, 经颅磁刺激品牌, 英国经颅磁刺激, 英国magstim, 经颅磁刺激仪, magstim, 经颅磁刺激, 经颅磁, 进口重复经颅磁刺激仪